Home » Research Advances

Abstract
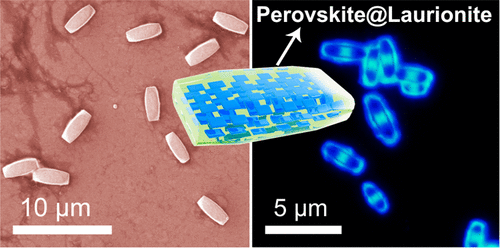
Reduced dimensional lead halide perovskites (RDPs) have attracted great research interest in diverse optical and optoelectronic fields. However, their poor stability is one of the most challenging obstacles prohibiting them from practical applications. Here, we reveal that ultrastable laurionite-type Pb(OH)Br can spontaneously encapsulate the RDPs in their formation solution without introducing any additional chemicals, forming RDP@Pb(OH)Br core�Cshell microparticles. Interestingly, the number of the perovskite layers within the RDPs can be conveniently and precisely controlled by varying the amount of CsBr introduced into the reaction solution. A single RDP@Pb(OH)Br core�Cshell microparticle composed of RDP nanocrystals with different numbers of perovskite layers can be also prepared, showing different colors under different light excitations. More interestingly, barcoded RDP@Pb(OH)Br microparticles with different parts emitting different lights can also be prepared. The morphology of the emitting microstructures can be conveniently manipulated. The RDP@Pb(OH)Br microparticles demonstrate outstanding environmental, chemical, thermal, and optical stability, as well as strong resistance to anion exchange processes. This study not only deepens our understanding of the reaction processes in the extensively used saturation recrystallization method but also points out that it is highly possible to dramatically improve the performance of the optoelectronic devices through manipulating the spontaneous formation process of Pb(OH)Br.
KEYWORDS:
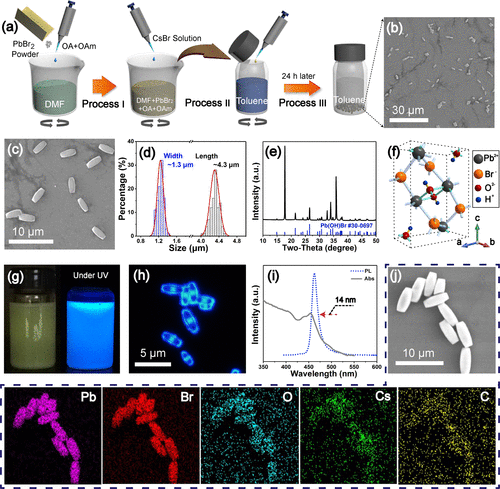
Figure 1. Synthesis and characterization of RDP@Pb(OH)Br microbricks. (a) Schematic of the preparation process. (b,c) SEM images of the microbricks with different magnifications. (d) The width and the length distribution of the microbricks. (e) XRD pattern. (f) The crystal structure of Pb(OH)Br. (g) The photos of the dispersed microbricks under white (right) and UV light (left) irradiation. (h) Fluorescence microscope image under UV excitation. (i) The PL (dashed line) and the absorption spectrum (solid line) of the microbricks on a piece of glass slide. (j) EDS element mapping results.
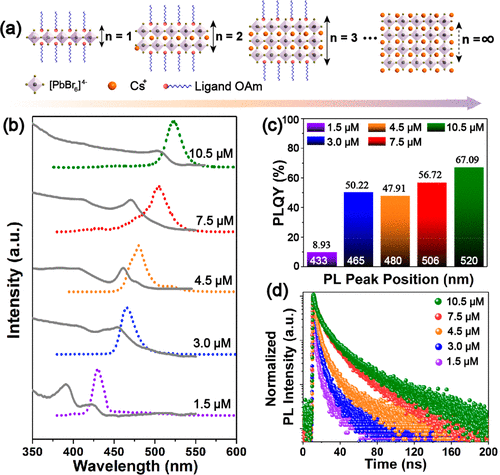
Figure 2. Perovskite layer number control of RDPs within Pb(OH)Br. (a) Schematic of the RDPs composed of ⟨n⟩ layers of perovskite. (b) The evolution of the PL (dotted lines) and the absorption spectra (solid lines) as the amount of CsBr was increased in the reaction solution. (c) The PLQYs of the RDP@Pb(OH)Br microbricks prepared by introducing different amounts of CsBr. (d) The PL decay spectra of the RDP@Pb(OH)Br microbricks prepared by introducing different amounts of CsBr.
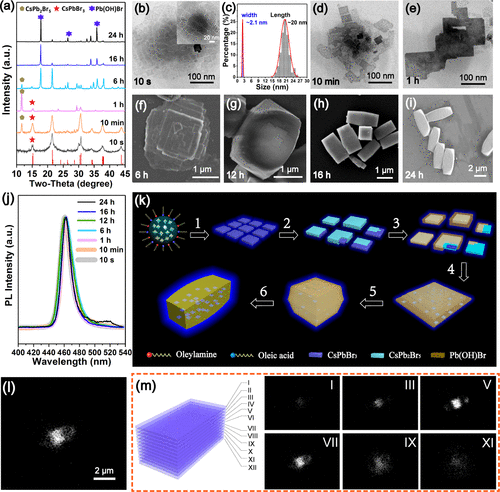
Figure 3. Growth process of the RDP@Pb(OH)Br microbricks. (a) The evolution of the XRD patterns as the reaction proceeds. (b�Ci) The morphology of the products at different reaction times. (j) The evolution of the PL spectra as the reaction proceeds. (k) Schematic of the growth process of the RDP@Pb(OH)Br microbricks. Process 1: Formation of RDP nanoflakes. Process 2: Growth of Cs2PbBr5 surrounding the nanoflakes. Process 3: Growth of Pb(OH)Br surrounding the nanoflakes. Process 4: The nanoflakes assemble into large microplates. Process 5: Microcuboids form through layer-by-layer stacking of the microplates. Process 6: Formation of microbricks through elongation growth of the microcuboids. (l) Integrated PL intensity mapping of a single RDP@Pb(OH)Br microbrick. (m) Schematic of the ��sliced�� layer and the brightness reflected the PL intensity at different ��sliced�� layer.
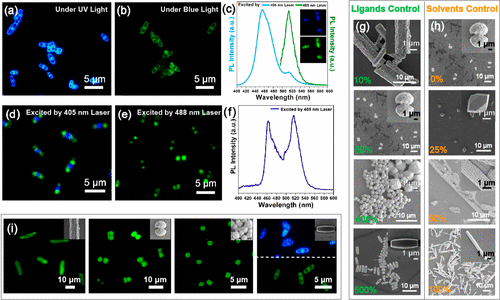
Figure 4. Engineering excitation wavelength-dependent and color barcode RDP@Pb(OH)Br microbricks. (a,b) Fluorescence microscope image of the RDP@Pb(OH)Br microbricks under UV and blue light excitation. (c) PL spectra of the RDP@Pb(OH)Br microbricks under 405 and 488 nm laser excitation. Inset: Confocal fluorescence microscope image under 405 nm (top) and 488 nm (bottom) laser irradiation. (d) Color barcode RDP@Pb(OH)Br microbricks with ends emitting a green color while the middle part emits a blue color under 405 nm laser irradiation. (e) Color barcode microbricks under 488 nm laser irradiation with only two ends emitting green light. (f) PL spectrum of the color barcode microbricks under 405 nm laser excitation. (g) Microparticles with different morphologies formed at different volume ratios of OAm to OA. The volume of OA was fixed at 75 ��L. Inset: Enlarged observation. (h) Microparticles formed at different volume ratios of DMSO to DMF. The total volume of the mixed solvent was fixed at 750 ��L. Inset: Enlarged observation. (i) Microparticles with typical morphologies with different light emissions. Inset: Corresponding microparticles.
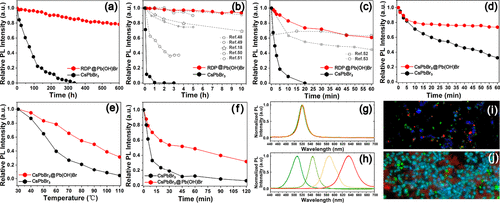
Figure 5. Stability improvement of the RDPs and CsPbBr3 nanocubes by Pb(OH)Br encapsulation. (a) The PL intensity evolution of the RDP@Pb(OH)Br microbricks and the ��naked�� CsPbBr3 nanocubes when preserved at ambient conditions. (b) The PL intensity evolution of the RDP@Pb(OH)Br microbricks and the ��naked�� CsPbBr3 nanocubes when stored in water. (c) The PL intensity evolution of the RDP@Pb(OH)Br microbricks and the ��naked�� CsPbBr3 nanocubes when incubated in ethanol. For comparison, experimental results from previous studies were included in (b,c). (d) The PL intensity evolution of the CsPbBr3@Pb(OH)Br microbricks and the ��naked�� CsPbBr3 nanocubes when irradiated by a 365 nm lamp (12 W). (e) The PL intensity evolution of the CsPbBr3@Pb(OH)Br microbricks and the ��naked�� CsPbBr3 nanocubes after thermal treatment for 1 h at different temperatures. (f) The PL intensity evolution of the CsPbBr3@Pb(OH)Br microbricks and the ��naked�� CsPbBr3 nanocubes after thermal treatment at 80 ��C for different times. (g,h) The PL spectra of CsPbBr3@Pb(OH)Br microbricks and ��naked�� CsPbBr3 nanocubes when exposed to iodine salt (OAm-I) solutions. (i,j) Fluorescence microscope image of green and blue light-emitting RDP@Pb(OH)Br particles mixed with red light-emitting CsPbBrxI3�Cx nanoparticles and embedded in a red light-emitting CsPbBrxI3�Cx thin film, respectively.
Conclusion
In summary, we have demonstrated that laurionite-type Pb(OH)Br could be spontaneously grown surrounding RDPs in their formation solution without introducing any additional chemical agents. The number of the perovskite layers in the RDP nanoflakes within Pb(OH)Br could be simply adjusted by varying the amount of CsBr introduced into the reaction solution, which can be varied from two to infinite corresponding to the PL emission from 430 to 522 nm. RDPs with different numbers of perovskite layers could coexist within the Pb(OH)Br microparticles, demonstrating excitation wavelength-dependent PL properties. More interestingly, color barcoded microparticles were prepared through controlling the location of the RDPs with different numbers of perovskite layers. The morphology of the RDP@Pb(OH)Br microparticles could be manipulated by varying the amount/type of the capping agents or the solvents. The ultrastable Pb(OH)Br shell could protect the inside RDPs from being influenced by air, water, and ethanol. Halide exchange reactions normally taking place for lead halide perovskites could also be prohibited by the Pb(OH)Br shells. The photostability and thermal stability of the CsPbBr3 nanocrystals were also significantly improved by the Pb(OH)Br protection. Overall, laurionite-type Pb(OH)Br could grow surrounding RDPs in their formation solution, forming RDP@Pb(OH)Br core�Cshell microparticles. These microparticles have exciting application potential in imaging and informatics fields benefiting from their unique optical properties and outstanding stability.
Information��
Ultrastable Laurionite Spontaneously Encapsulates Reduced-dimensional Lead Halide Perovskites[J]. Nano Letters, 2020, 20(4): 2316−2325.
Yangchun Yu#, Jiahui Hou#, Linghui Zhang, Qisheng Sun, Sanam Attique, Weijian Wang, Xiuxia Xu, Fan Xu, Zhipeng Ci, Bingqiang Cao, Xvsheng Qiao, Xiangheng Xiao and Shikuan Yang
DOI: 10.1021/acs.nanolett.9b04730.
https://pubs.acs.org/doi/10.1021/acs.nanolett.9b04730

